Determination of FeNO NIOX VERO®
-
in Diagnostics
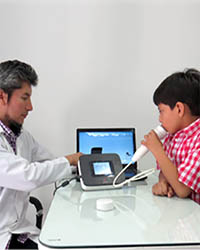
NIOX VERO® FeNO — determination of the level of nitrogen oxide in exhaled air
Today, nitric oxide (NO) is known as a biological mediator in the body of animals and humans. In humans, NO is produced in the lungs and is present in exhaled air. It is involved in the pathophysiology of lung diseases, including bronchial asthma (BA). Measurement of exhaled NO has been standardized for clinical use. The use of NO measurement in clinical practice has a large evidence base.
FeNO is an objective biomarker of inflammation in the respiratory tract
Fraction of exhaled nitric oxide (FeNO) is a quantitative, noninvasive, simple, and safe measure of airway inflammation that provides an additional tool to assess airway inflammation in patients with asthma.

In particular, FeNO is an objective indicator of allergic/eosinophilic inflammation. Airway inflammation occurs as a result of the activation of mast cells and antigen-specific T helper type 2 (Th2), which leads to the production of cytokines, including interleukin (IL)-4, IL-5, and IL-13.3. This is an important distinction because Th2 allergic inflammation and eosinophilic inflammation are not the same thing. However, they are very closely related to similar molecular pathways and crossovers.
Measurement of FeNO with the NIOX VERO can help in the treatment of patients:
- When diagnosing asthma and identifying patients with Th2 allergic/eosinophilic inflammation
- Determine the response to steroid treatment and optimize the dose of inhaled steroids
- Identify non-adherence to inhaled corticosteroids
- Reduce the likelihood of exacerbations in patients with high risk and severe asthma
- To identify patients with asthma who are possible candidates for treatment with biologics
Routine monitoring of FeNO helps in optimizing treatment
NIOX VERO gives you an objective and accurate assessment of airway inflammation right at the allergist's appointment
- Establish the patient's baseline and best personal FeNO level 2.3
- Assess adherence to treatment with inhaled corticosteroids (ICS) 4.5
- Optimize the dose and use of ICS 4.6
- Identify patients with asthma who are possible candidates for treatment with biologics
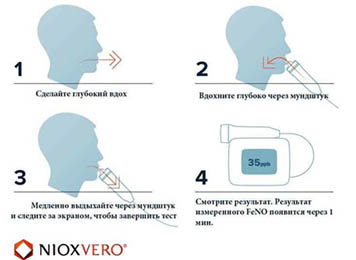 Methodology of testing
Methodology of testing
МThe method is performed for children from 5 years of age and adults. The duration of the study takes several minutes. Exhalation itself lasts 6 seconds for children and 10 seconds for adults. Exhalation data can be presented on the computer monitor in the form of animation (a cloud, a girl with a balloon), which additionally attracts the attention of children, relieves tension in the doctor's office and becomes practically a game procedure.
Preliminary preparation:
- Avoid drinking alcohol 2 days before the study.
- Antihistamines, inhaled corticosteroids, and bronchodilators should not be discontinued.



